Table of Contents
ToggleUnderstanding the Melting Point of Plastics
The melting point of plastics refers to the temperature range at which plastics transition from a solid to a flowable liquid state. At this point, plastics soften and become pliable, making them suitable for various molding and shaping processes.
Thermoplastics vs. Thermosets
Plastics can be categorized into two main types: thermoplastics and thermosets.
Thermoplastics soften upon heating and harden upon cooling, a reversible process that allows for repeated heating and cooling without altering the material’s properties.
Thermosets, on the other hand, undergo a chemical reaction upon initial heating, resulting in a permanent, non-melting network structure. Even upon subsequent heating, they do not soften.
So, in our subsequent discussions about melting points, the plastics we mention will all be thermoplastics.
Important for Injection Molding, Extrusion and Formation
The process of softening and melting plastics is crucial in plastic processing.
- During injection molding, precise control of the melting temperature ensures that the plastic fully occupies the mold and achieves the desired shape.
- The extrusion process takes advantage of the flowability of heated plastics to produce pipes and profiles using specific molds.
- The formation of films or sheets also depends on the plastic’s ability to deform plastically at the appropriate temperatures.

Therefore, for designers and processors, understanding the working temperature and optimal processing temperature of plastics is vital for controlling product quality and meeting application requirements.
Melting Characteristics of Plastics
Crystalline vs. Amorphous Materials
Materials in nature are divided into crystalline and amorphous.
Crystalline materials have orderly arranged molecules or atoms, possessing fixed melting points. For example, water melts at 0°C, salt (NaCl) at 801°C, and tin at 231.9°C.
On the other hand, amorphous materials, including glass, rubber, plastics, asphalt, rosin, and paraffin, have molecules or atoms arranged in a disordered manner and lack a fixed melting point. When heated, they typically soften (rubbery state) before liquefying (viscous flow state), spanning a certain temperature range instead of melting at a specific point.
Types of Plastics: Amorphous and Crystalline
Amorphous Plastics:
Under typical processing conditions, these plastics do not have crystalline regions and are entirely amorphous. Examples include polycarbonate, ABS, PMMA, ASA, PPSU, etc. Their melting behavior aligns with that of typical amorphous materials.
Crystalline Plastics:
Many plastics tend to crystallize as they cool and solidify, such as polyethylene (PE), polypropylene (PP), polyoxymethylene (POM), polyamide (PA6 and PA66), PET, and PBT.
However, they crystallize only in certain regions, with materials having a crystallinity higher than 80% classified as crystalline plastics and the rest as semi-crystalline.
The degree of crystallinity is greatly influenced by the cooling process; slow cooling within the crystallization temperature range can increase crystallinity, whereas rapid cooling has the opposite effect.
Thus, the melting process of these crystalline plastics partially resembles that of crystalline materials but also incorporates characteristics of amorphous materials.
The Three States and Four Key Temperatures of Plastics During Heating
Let’s explore the three states that plastics undergo during heating: the glassy state, the high-elastic (rubbery) state, and the viscous flow state, as well as the four key temperatures associated with them: the glass transition temperature, melting temperature, flow temperature, and decomposition temperature.
The Glassy State
The first state is the glassy state, where plastics are at room or low temperatures. In this state, the movement of plastic molecules is greatly restricted, making the material rigid and brittle. This occurs because the temperature is below the glass transition temperature (Tg), where the intermolecular forces are greater than the thermal energy, preventing free molecular movement.
Glass Transition Temperature (Tg) and High-Elastic (Rubbery) State
A significant physical change occurs when plastics are heated to the glass transition temperature. Tg marks the start of the transition from a hard and brittle state to a soft state, with the exact temperature depending on the type of plastic and its molecular structure.
For example, Polyoxymethylene (POM) has a Tg of around 85°C, while Polycarbonate (PC) has a higher Tg, usually around 145°C.
Above Tg, polymer chains gain more energy and start to move more freely, yet they remain in a randomly disordered state. As the temperature continues to rise, plastics enter the high-elastic state, also known as the rubbery state. In this state, plastics show significant elasticity and flexibility. The movement between polymer chains increases, but there is still some degree of intermolecular interaction. Plastics in this state can undergo significant deformation without breaking, ideal for many rubber products and flexible plastic items.
Flow Temperature (Tf) and Viscous Flow State
Finally, as the temperature further increases to the flow temperature (Tf), plastics enter the viscous flow state. In this state, plastics become more fluid, similar to a viscous liquid. In the viscous flow state, plastics can be extruded, injected, or compressed into shapes. This is the key stage of plastic processing, such as injection molding which is done in this state.
During the barrel purging process, the molten state of the plastic can be clearly observed.
Decomposition Temperature (Td)
The last significant stage in the heating of plastics is the decomposition temperature (Td). This is the point where plastics start to chemically decompose, losing their original physical and chemical properties. Upon reaching or exceeding the decomposition temperature, plastics begin to break down into smaller molecules, potentially releasing gases and other decomposition products. Therefore, it is essential to avoid surpassing the decomposition temperature during processing to prevent material damage and the possible release of harmful substances.
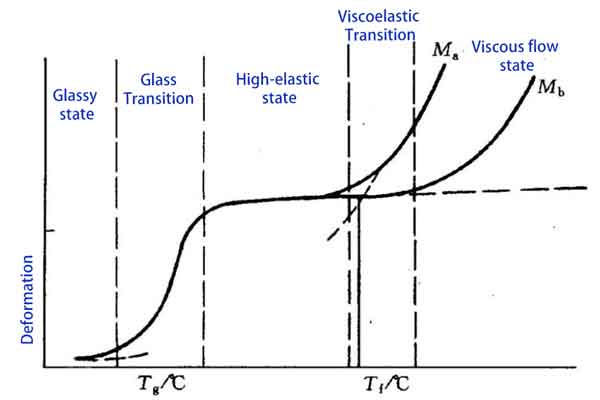
Note:
- Ma: Amorphous region
- Mb: Semi-crystalline region
Tip: The Melting Temperature (Tm), Also Known as Flow Temperature (Tf)
It’s important to note that the melting temperature of plastics is not a fixed point, but a range. Within this range, the physical state of plastic transitions from the high-elastic state to the viscous flow state. For instance, the melting temperature range for Polypropylene (PP) is from 160°C to 175°C, while for Polyethylene (PE), it is from 125°C to 137°C. The width of this range depends on the specific type of plastic and the complexity of its molecular structure.
Melting Temperatures of Common Plastics
Here, we have listed the melting temperatures, injection molding temperatures, and decomposition temperatures of some common plastic materials. It’s important to note that the injection molding temperature is usually higher than the melting temperature to ensure good flowability of the plastic during processing.
Because there are many types of modified plastics and their properties are very different, it is impossible to list too many materials in this table. For example, the temperature characteristics of nylon with added glass fibers differ significantly from those of nylon without glass fibers. In practice, it’s easy to obtain a material’s property sheet when purchasing plastic materials. Therefore, this table is intended only as a rough reference.
Common plastics’ melting temperatures
| Material Name | Melting Temperature (°C) | Injection Molding Temperature (°C) | Decomposition Temperature (°C) |
|---|---|---|---|
| ABS | 170-190 | 200-240 | 280 |
| PP (Polypropylene) | 160-175 | 190-290 | 320 |
| POM (Polyoxymethylene) | 165-175 | 190-230 | 280 |
| PC (Polycarbonate) | 225-250 | 270-320 | 360 |
| PBT | 225-235 | 220-270 | 280 |
| PA6 (Nylon 6) | 215-221 | 260-300 | 320 |
| PA66 (Nylon 66) | 260-265 | 270-310 | 360 |
| PMMA (Acrylic) | 160-180 | 220-250 | 270 |
| LDPE (Low-Density Polyethylene) | 110-130 | 150-230 | 300 |
| HDPE (High-Density Polyethylene) | 125-137 | 160-280 | 300 |
| PEEK (Polyether Ether Ketone) | 315-353 | 360-400 | 520 |
This table provides an overview of the melting, injection molding, and decomposition temperatures of various common plastics.
Conclusion
In conclusion, understanding the melting, injection molding, and decomposition temperatures of plastics is essential in the field of material science and manufacturing. These temperatures not only guide the processing and application of plastics but also ensure the safety and quality of the final products.
As the industry evolves with new materials and technologies, continued research and knowledge in this area remain crucial. Whether in industrial applications or daily use, the versatile nature of plastics continues to shape the modern world.

